Lista de encabezados
Voluntary Urgent Medical Device Correction Notice for FreeStyle Libre

Descripción
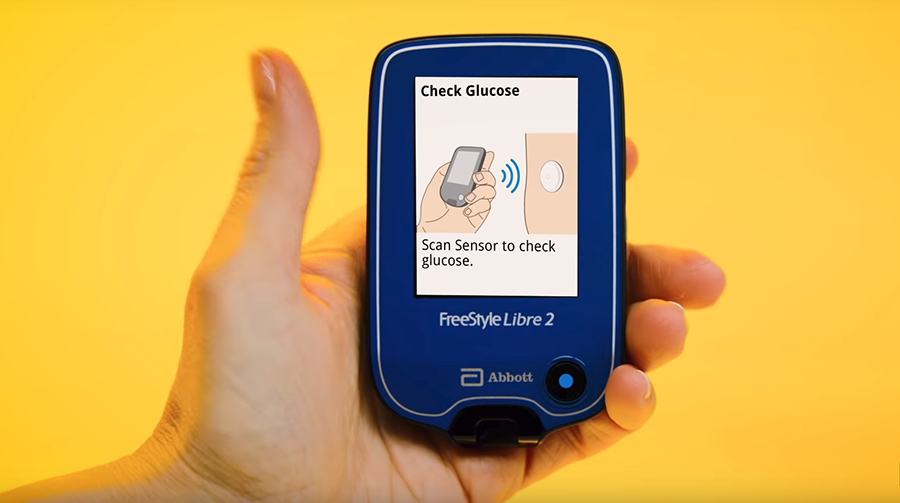
Abbott Freestyle Libre 2 Gets CE Mark
Drug Recalls
Drug Recalls

PDF) Budget Impact Analysis of the FreeStyle Libre Flash Continuous Glucose Monitoring System® in Patients with Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus with Multiple Daily Insulin Injections in Argentina

FDA Issues Safety Notice for Freestyle Libre Glucose Monitoring Readers
Voluntary Urgent Medical Device Correction Notice for FreeStyle Libre
Flash Glucose Monitoring System - Scan the Sensor for Glucose Reading - Real Time Glucose Alarms - For Single Person Use Only - For Use with FreeStyle
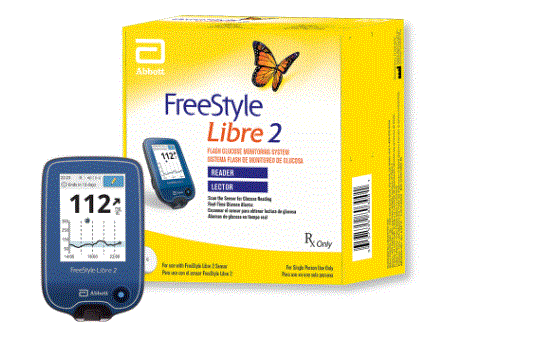
Libre 2 Reader (57599-0803-00)

Alerts

FreeStyle Libre 14-Day Flash Glucose Replacement Sensor — Mountainside Medical Equipment
Sugerir búsquedas
€ 8.50EUR
puntaje 4.9(403)
En stock
Continuar reservando
€ 8.50EUR
puntaje 4.9(403)
En stock
Continuar reservando
©2018-2024, shabakekaraniran.ir, Inc. o sus afiliados







